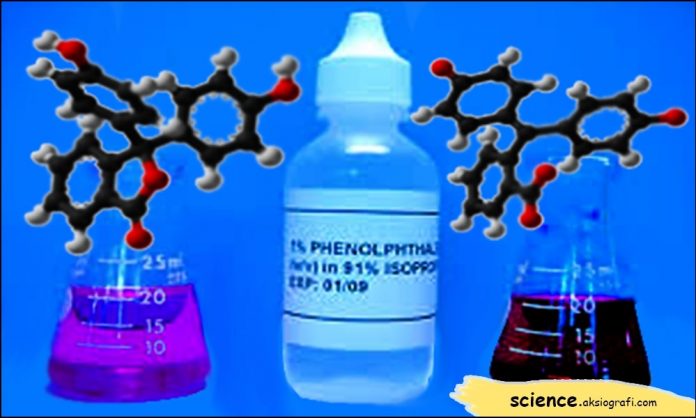
How to make phenolphthalein as an indicator. As an indicator in acid-base titration, Phenolphthalein changes color in the test. From colorless in an acidic solution to an easy red color in alkaline solutions.
Science.aksiografi.com – Phenolphthalein is a dye that functions as an indicator of pH (Power of Hydrogen), the degree of acidity. Phenolphthalein is a chemical compound, in the molecular formula C20H14O4. Phenolphthalein, in writing, is often shortened to “Hln” or “pp”.
Phenolphthalein, in particular, determines the presence of bases because when mixed with hydroxide ions, their chemical structure changes, and reflects a pink color. In a neutralizing reaction, the acid or base reacts to form a salt and water.
As an indicator in acid-base titration, Phenolphthalein changes color in the test. From colorless in an acidic solution to an easy red color in alkaline solutions. In performing either laboratory or independent experiments, Fln is dissolved in alcohol.
In this experiment, we will try to make a chemical solution of Phenolphthalelin as an indicator to be used as a test material in the School of Nature.
Materials
4 tablespoons (60 ml) of rubbing alcohol
2 baby-food jars (one with a lid)
1 Ex-Lax® tablet
spoon
Procedure
1. Pour the rubbing alcohol into the first jar.
2. Break the Ex-Lax tablet into small pieces and place them in the jar of alcohol.
3. Use the spoon to crush and stir the tablet pieces. Note: The tablet will not dissolve completely. Pieces will settle to the bottom of the solution.
4. Decant the clearer liquid at the top into a clean jar.
5. Secure the lid on the jar to store.
Result
Practice how to make phenolphthalein works. The solution in ready for use as a test material in the Nature School practice.
Why?
Normality (N) is a concentration unit used to measure the number of equivalents (quantities of substances that have the same combining capacity) per liter of solution. The number of equivalents of an acid or a base can be calculated by multiplying the normality of a solution times its volume in liters. Adding a measured amount of a base to a measured amount of an acid is a laboratory process called titration. During titration, the acids and bases chemically react in equivalent amounts. Note: As long as both the acid and base have the same volume unit any measuring unit may be used including drops.
Example: In a titration, 30.0 ml of 0.04 N ammonium hydroxide is added to 20.0 ml of hydrochloric acid. What is the normality of the hydrochloric acid? Note: Since the units of both volume measurements are milliliters, there is no need to change them to liters. Also, the equation that follows uses the symbols N for normality and V for volume.
N (acid) x V (acid) = N (base) x V (base)
N (acid) x 20.0 ml = 0.04 N x 30.0 ml
N (acid) = 0.04 N x 30.0 ml / 20.0 ml
= 0.06 N